
Postdoctoral Fellow


Laboratory of TIME
(Tumour Immune Microenvironment)
Cancer patients show differential responses to existing chemotherapy and immunotherapy regimens due to the presence of complex tumour immune microenvironments (TIME). This complex TIME features immune cells, stromal cells, nerve cells, blood vessels and extracellular matrix that maintain cordial relationships with cancer cells, supporting their survival, growth and spread. Multiple secretory factors, including chemokines, cytokines, and metabolites, build an immunosuppressive tumour microenvironment (TME) by inhibiting the T cell-mediated antitumour immunity and by enhancing the infiltration of immunosuppressive tumour-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and T-regulatory cells (Treg). Therefore, there is always a tug-of-war between immunosuppression dominated by tumour-associated macrophages, T-regulatory cells, myeloid-derived suppressor cells, and T-cell immunity that includes activated dendritic cells and cytotoxic T cells.
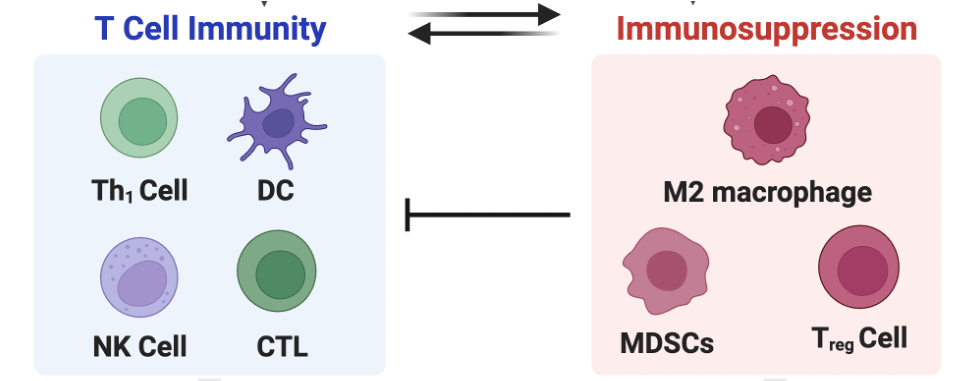
We aim to build multiple strategies that can tilt the balance of this tug-of-war towards T cell immunity against cancer, especially breast and colorectal (CRC) cancer.
To build these strategies, we are running the following programs:
Deciphering the Role of Cancer Metabolism on TIME
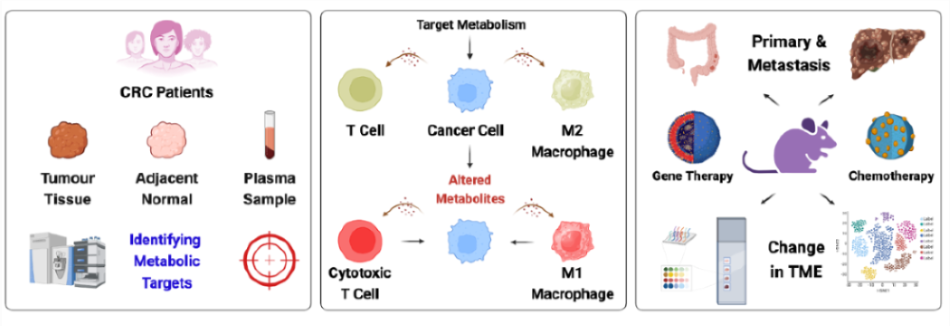
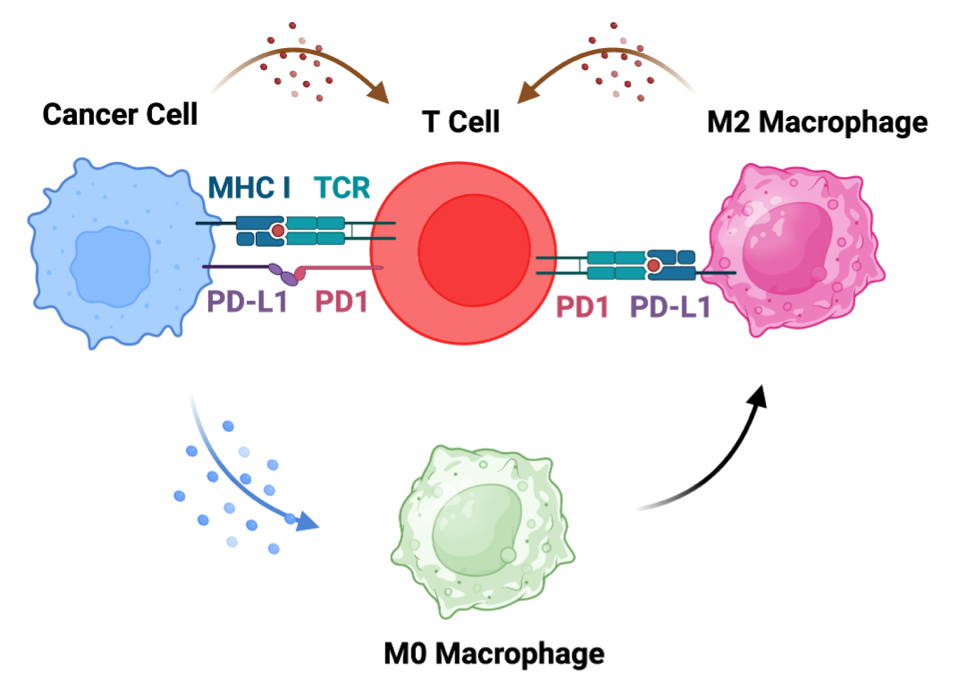
One of the major challenges to the poor efficacy of the current therapeutic regimens for cancer treatment is the immunosuppressive TIME that inhibits the efficacy of chemotherapy and immunotherapy regimens. Another major challenge is the inability of the current therapeutic regimens to target metastatic tumours. Therefore, there is a need to develop therapeutic regimens that can mitigate immunosuppressive TIME and clear metastatic tumours. In addition to the genomic, phenotypic, and molecular diversity observed among various cancer types, metabolism has emerged as a key hallmark influencing the pathophysiology of cancer, particularly following the identification of the Warburg effect. Recent insights into tumour metabolism indicate that metabolic alterations not only influence the activation of oncogenes and the inactivation of tumour suppressor genes but also contribute to immunosuppression by inhibiting T-cell activity and diminishing the effectiveness of immune checkpoint blockade therapy. Therefore, we aim to decipher the impact of altered cellular metabolism on TIME and identify the key therapeutic targets for modulating TIME.
Elucidating the Role of Neurons on TIME
Neurons crosstalk with cancer cells and other stromal cells, including immune cells, in the TME through neurotrophic factors, neuropeptides, and neurotransmitters. Neurotrophic factors and axon guidance molecules, including NGF (nerve growth factor), BDNF (brain-derived neurotrophic factor), netrin-1, and EphrinB1 released from cancer cells boost nerve density in tumour tissues by promoting the outgrowth of nerves. These neurons interact with cancer cells and immune cells by secreting neurotransmitters, including noradrenaline, acetylcholine, and CGRP (calcitonin gene-related peptide), from sympathetic, parasympathetic and sensory neurons, respectively. Therefore, in this program, we aim to decipher the impact of neurons on TIME and identify the cross-talk between neurons and cancer/immune cells to identify new therapeutic targets.
Investigating the Impact of TIME on Chemoresistance
Chemoresistance presents a significant challenge in contemporary cancer therapy, frequently resulting in tumour recurrence and metastasis. TIME, which involves cytokines, immune cells, and immunomodulators, is known to influence the treatment response in cancer patients. However, little is known about how changes in the TIME impact tumour growth and therapeutic resistance. Therefore, we aim to understand the intricate relationship between TIME and cancer chemoresistance. In particular, we are interested in how metabolic reprogramming in resistant cells, beyond promoting tumour growth and progression, suppresses T-cell function, influences immune evasion, and compromises immunotherapeutic responses. Therefore, we aim to dissect the molecular, metabolic, and immunological changes in resistant tumours to discover possible targets for boosting anti-tumour immunity and overcoming the chemoresistance challenge, to open a new avenue for more durable and comprehensive anti-cancer therapies.
Elucidating the Role of Mycobiome on TIME
Tumour-associated microbiota is the specific microbiome composition in the TME, and it varies in composition from healthy people. Tumour-associated microbiota has been reported in the TIME of different cancers and has been shown to play a role in tumour progression, metastasis and immunosuppression. Metabolite-mediated cross-talk between gut microbiome and cancer/immune cells provides crucial therapeutic targets for colon cancer. Therefore, we aim to investigate the causal relationship and mechanistic pathways to understand the role of microbiota, especially the mycobiome dysbiosis, in cancer patients for developing futuristic therapeutic strategies.
Publications from Regional Centre For Biotechnology
Publication from University of Massachusetts, Amherst, USA.
Publications from Indian Institute of Science, Bangalore
Former PhD Students
Former Postdoctoral Fellows
Former Research Fellows
Former Trainees
Regional Centre for Biotechnology
NCR Biotech Science Cluster
3rd Milestone, Faridabad-Gurgaon Expressway
P.O. Box No. 3, Faridabad - 121 001
Haryana (NCR Delhi), India
E-mail: bajaj[at]rcb[dot]res[dot]in
Phone: 91 129 2848831